Medical Devices
Our medical device testing experts assess the strength, resistance, durability and ageing of various types of joint endoprosthesis, osteosynthesis products, trauma products, surgical instruments and tissue adhesives. We also provide manufacturing and development services to help quickly get your medical devices on the market.
We hold the required accreditations to test the materials, components and complete devices to the major international test standards, and we also develop new test procedures for innovative products including where no relevant test standards could apply.
Medical Device Testing: Our Methodology
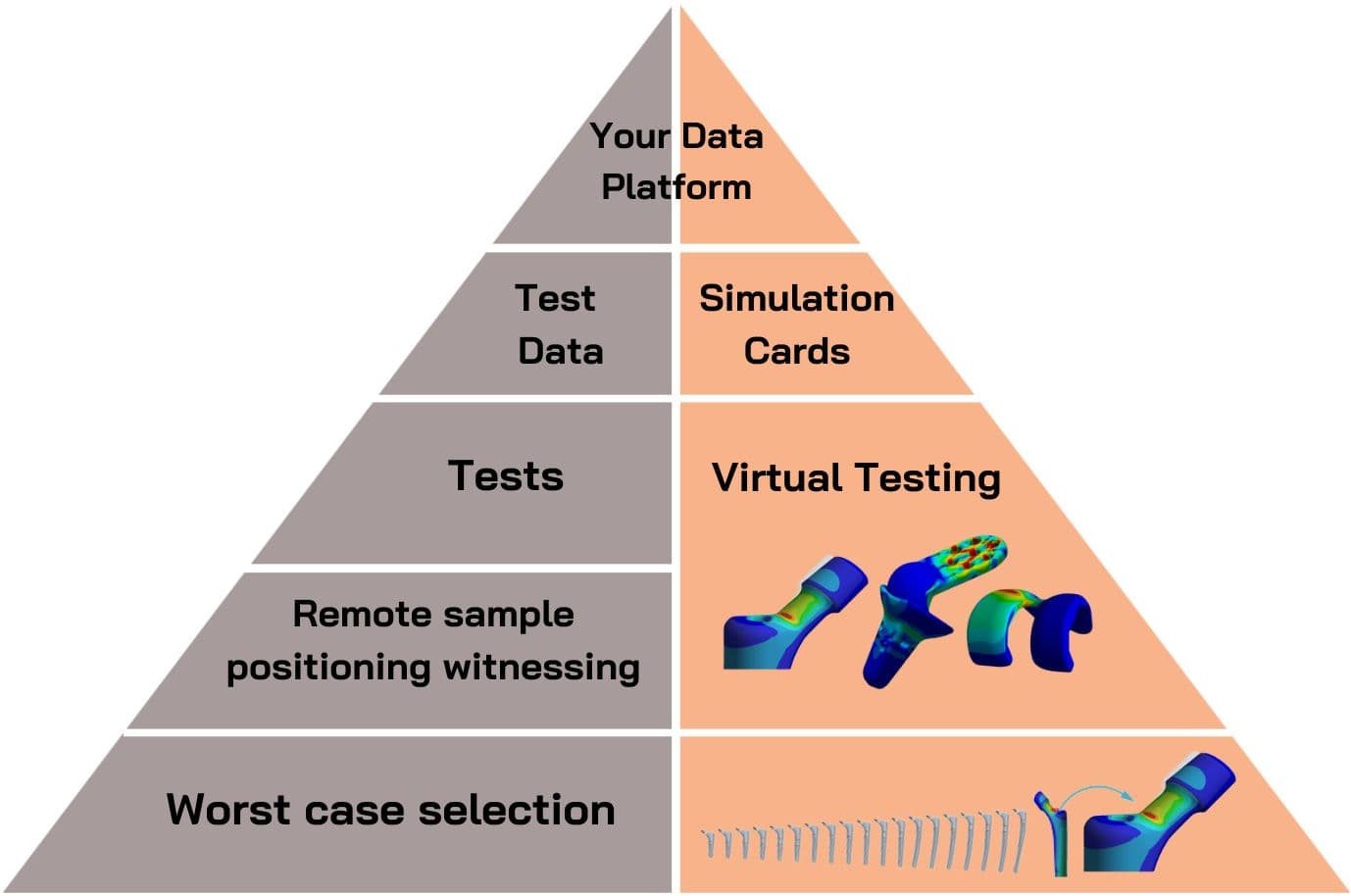
At Applus+ Laboratories, we pride ourselves on being able to provide a complete medical device testing service. Our objective is to offer our clients a full one-stop-shop for testing, developing, and ultimately marketing their medical device innovation and to ensure they receive the highest quality service while we do so.
Here’s a breakdown of our carefully crafted medical device testing journey, from simulation and strength assessments all the way to cleaning and packaging validation.
Simulation and Strength Assessments for Medical Devices
Simulation and strength assessments are crucial in the development of your medical device, ensuring that it is fully optimised even during its design stage.
With material efficiency and design innovative pushing the boundaries, our medical device testing experts at Applus+ Laboratories perform an extensive range of tests so that even the more innovative designs can break into the market as quickly as possible.
What Simulation and Strength Assessments Do We Perform?
In our state-of-the-art testing facilities, we conduct rigorous medical device testing, including:
- Simulation of contact situations
- Simulation of interference fits
- Simulation of hyperelastic materials and plastics
- Lifetime analyses of the structure
Our strength assessments also include evaluating the worst-case geometric variant of your medical device to reduce costs and testing times. This means we fully test your product’s endurance even at its most extreme.
Finite Element Analysis (FEA) for Medical Device Testing
Applus+ Laboratories also offers Finite Element Analysis (FEA) to assess your medical device’s dimensions, aiming to find the optimal mass and shape, offering our expert testing recommendations on how to improve your device. FEA includes:
- Dimensioning of the product
- Optimisation of mass, shape
- Determination of stresses, deformations
- Investigation stability behaviour
- Static, operational and fatigue strength verifications
Medical Material and Coating Testing
In addition to design, Applus+ Laboratories also provides medical material and coating testing to assess their suitability for long-term endurance after implantation. Testing materials and coating involves analysing material samples as well as finished implants and surgical instruments ensure an increasingly reliable product for the patient.
At Applus+ Laboratories, we are accredited to carry out materials tests for metals, plastics and ceramics and on all coatings.
Medical Tests on Implants and Biomaterials
Applus+ Laboratories performs full lifecycle testing on implants as well as comprehensive biomaterials testing. These tests are critical for confirming the lifecycle of an implant by simulating how it can withstand wear and tear over long-term use. Applus+ Laboratories is fully accredited to perform all the necessary testing to get your implant to market as soon as possible.
Here’s an overview of the medical devices we test on:
Implant Testing for Medical Devices
Joint implants are loaded for up to ten million cycles in the anatomically correct position under physiologic conditions in our laboratories, and we perform tests that simulate the full lifecycle of an implant. Here’s a full list of the testing services we provide:
- Wear Testing: Evaluates how materials withstand friction and abrasion, predicting their durability in real-world applications.
- Fatigue Testing: Assesses material performance under repeated stress cycles for tension, pressure, torsion, and shear, ensuring they can endure long-term usage without failing.
- Corrosion Testing: Examines material degradation in harsh environments, crucial for predicting longevity and safety against corrosion.
- Contact Area/Stress Test: Measures pressure distribution and stress at contact points, helping optimize design and performance under load.
- Simulation and Strength Assessment: Uses computational models to predict how materials will respond to various forces, aiding in design and safety evaluations.
- Mechanical and Physical Tests on PE: Tests the physical and mechanical properties of polyethylene (PE) materials, ensuring they meet specific performance criteria.
- Materialography – Microstructure Analysis: Analyses the microstructure of materials to understand their composition and predict their behaviour under different conditions.
- Damage Analysis: Investigates the causes and extent of material damage, providing insights for improving durability and preventing future failures.
- Materials and Coatings: Evaluates the properties and effectiveness of materials and their protective coatings, ensuring they meet performance standards.
- Sample Preparation: Involves preparing materials for detailed analysis, ensuring accurate and reliable test results.
- Determination of Metal Ion Concentration: Measures the concentration of metal ions in materials, important for assessing contamination and compliance with safety standards.
- PE Particle Analysis: Analyses polyethylene (PE) particles to evaluate their size, distribution, and impact on material performance.
We also offer optional Artificial Aging of UHMWPE components according to ASTM F2003 for a duration of 2 weeks.
Product and Process Qualification – Batch Release Tests
After medical devices have undergone the vital strength and simulation Applus+ Laboratories provides the necessary product and process qualification culminating in batch release tests to ensure compliance and quality before market release. These involve several tests for chemical characterisation, biocompatibility, cleaning, packaging, and then stability:
Material and Medical Devices Chemical Characterisation
With material and chemical characteristics, the composition of the medical device’s materials is analysed and assessed to get an idea of what impact they may have on the human body. This involves breaking down the device to its material components, identifying whether there are any contaminants that could cause potentially harm.
Biocompatibility Testing
Applus+ Laboratories has extensive 20-year experience in Biocompatibility testing, essential for ensuring that a medical device does not result in adverse reactions or cause the human body harm due to the materials used in the device. Biocompatibility requires physicochemical characterisation of extractables and leachables, followed by a toxicological analysis and, if necessary, additional in vivo and in vitro tests to determine the biological risk of the medical device.
Cleaning Validation
In addition to biocompatibility testing, cleaning validation is also important to control the removal of residue or contaminating substances from a medical device to guarantee the effectiveness of its cleaning process.
Packaging Validation
Packaging validation assesses the effectiveness of medical device’s packaging, from ensuring it’s maintained sterile and sturdy enough to protect the product, as well as ensuring its safety during transport. Packaging validation is vital also for making sure that the medical device can be well-preserved during storage.
Stability Validation
Lastly, stability validation tests the product for its storage longevity. The medical device is tested under various conditions according to temperature and humidity exposure to see how well the device retains its quality and sterility during its shelf life.
Contract Manufacturing Organisation (CMO) for Medical Devices
Applus+ Laboratories is also a contract manufacturing organisation (CMO) that provides development services for your medical devices. These services include helping you with medical device discovery and development in order to bring your product to market as quickly and smoothy as possible.
At Applus+ Laboratories, we provide CMO services for:
- Orthopedics
- Spinal implants
- Dental implants
- Maxilocraneal implants
In addition, we also provide:
- Machining and milling
- Anodicing
- Laser marking
- Cleaning and packaging
Related services to Medical Devices
Applus+ uses first-party and third-party cookies for analytical purposes and to show you personalized advertising based on a profile drawn up based on your browsing habits (eg. visited websites). Click HERE for more information. You can accept all cookies by pressing the "Accept" button or configure or reject their use by clicking here.
-
Essential cookies
They allow the operation of the website, loading media content and its security. See the cookies we store in our Cookies Policy
Always active -
Analytics cookies
They allow us to know how you interact with the website, the number of visits in the different sections and to create statistics to improve our business practices. See the cookies we store in our Cookies Policy
-
Advertising cookies
Based on your behavior on the website (where you click, how long you browse, etc.) we establish parameters and a profile for you to display ads that correspond to your interests. See the cookies we store in our Cookies Policy